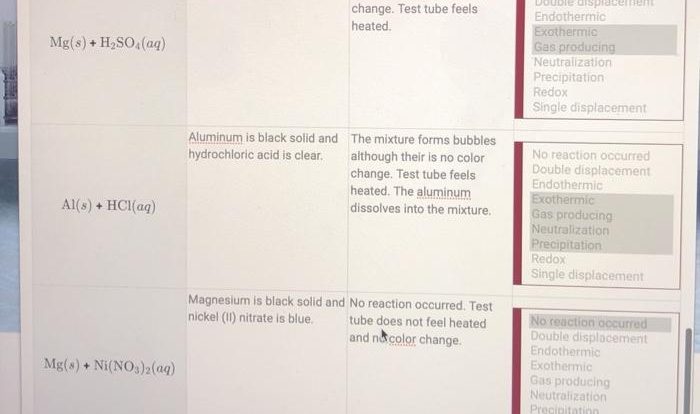
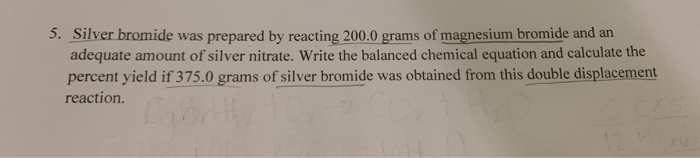Welcome to our empirical formula practice worksheet with answers! This resource has been meticulously crafted to provide you with a comprehensive understanding of empirical formulas, their significance, and their applications in chemistry.
Through a series of engaging and thought-provoking problems, this worksheet will guide you through the fundamental concepts of empirical formulas, enabling you to confidently determine the elemental composition of compounds and apply this knowledge to real-world scenarios.
Understanding Empirical Formula: Empirical Formula Practice Worksheet With Answers

An empirical formula represents the simplest whole-number ratio of atoms present in a compound. It provides crucial information about the compound’s elemental composition, but does not specify the actual number of atoms or the molecular structure.
For example, the empirical formula of glucose (C 6H 12O 6) indicates that for every six carbon atoms, there are 12 hydrogen atoms and six oxygen atoms. However, it does not specify the specific arrangement of these atoms within the glucose molecule.
Empirical formulas are particularly useful for comparing the elemental composition of different compounds and for determining the relative amounts of different elements present in a sample.
Limitations of Empirical Formulas, Empirical formula practice worksheet with answers
- Do not provide information about the molecular structure or bonding.
- May not be unique for compounds with the same elemental composition (isomers).
- Do not indicate the presence of ions or the oxidation states of atoms.
Common Queries
What is an empirical formula?
An empirical formula represents the simplest whole-number ratio of elements present in a compound, providing information about its elemental composition but not its molecular structure.
How is an empirical formula determined?
To determine an empirical formula, you need to know the mass or mole fractions of each element in the compound and then convert these values to the simplest whole-number ratio.
What are the limitations of empirical formulas?
Empirical formulas do not provide information about the molecular structure or the arrangement of atoms within a compound. They only indicate the relative proportions of elements present.