Divide the compounds below into electron-poor and electron-rich groups – In chemistry, understanding the electron distribution within compounds is crucial. This Artikel delves into the classification of compounds based on their electron richness or poverty, exploring their characteristics, factors influencing their electron distribution, and methods for distinguishing between them.
Electron-poor compounds are characterized by a deficiency of electrons, while electron-rich compounds possess an abundance of electrons. This distinction has significant implications for their chemical reactivity and behavior.
Electron-Poor Compounds
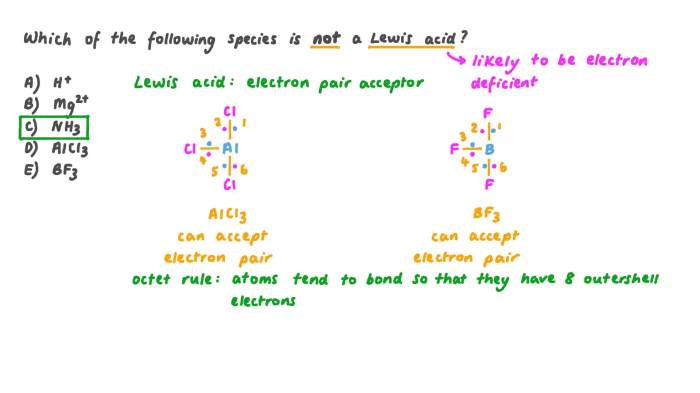
Electron-poor compounds are chemical species that have a deficiency of electrons. They are characterized by a positive charge or a high electronegativity, which makes them electron acceptors. Electron-poor compounds are often found in reactions where they can accept electrons from other molecules.
Characteristics of Electron-Poor Compounds
- Positive charge
- High electronegativity
- Electron acceptors
- React with electron-rich compounds
Examples of Electron-Poor Compounds
- Cations (e.g., Na+, Ca2+)
- Electrophilic molecules (e.g., CO2, SO2)
- Lewis acids (e.g., BF3, AlCl3)
Electron-Rich Compounds
Electron-rich compounds are chemical species that have an excess of electrons. They are characterized by a negative charge or a low electronegativity, which makes them electron donors. Electron-rich compounds are often found in reactions where they can donate electrons to other molecules.
Characteristics of Electron-Rich Compounds, Divide the compounds below into electron-poor and electron-rich groups
- Negative charge
- Low electronegativity
- Electron donors
- React with electron-poor compounds
Examples of Electron-Rich Compounds
- Anions (e.g., Cl-, OH-)
- Nucleophilic molecules (e.g., NH3, H2O)
- Lewis bases (e.g., pyridine, triethylamine)
Factors Affecting Electron Richness/Poorness
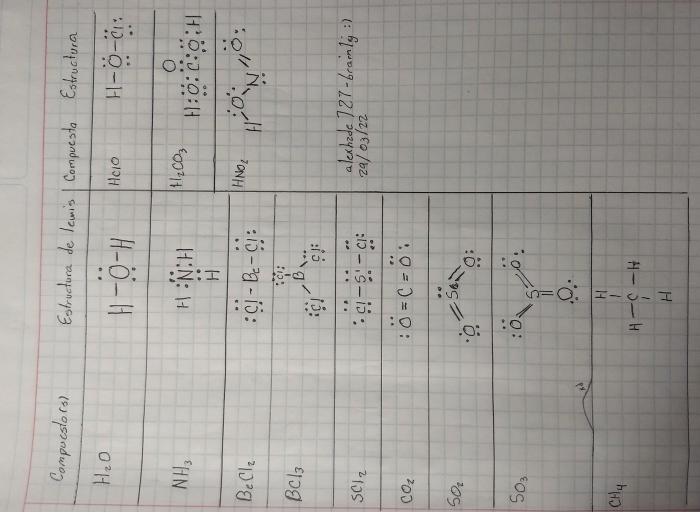
The electron richness or poorness of a compound is influenced by several factors:
Electronegativity
Electronegativity is the ability of an atom to attract electrons towards itself. Atoms with high electronegativity tend to form electron-poor compounds, while atoms with low electronegativity tend to form electron-rich compounds.
Bonding
The type of bonding between atoms can affect electron richness. Covalent bonds share electrons between atoms, while ionic bonds transfer electrons from one atom to another. Ionic bonds result in the formation of electron-poor and electron-rich species.
Resonance
Resonance is a phenomenon where multiple Lewis structures can be drawn for a molecule. Resonance can lead to the delocalization of electrons, which can make a compound more electron-rich or electron-poor.
Hybridization
Hybridization of atomic orbitals can also affect electron richness. Hybridized orbitals have different shapes and energies, which can influence the electron distribution in a molecule.
Methods for Distinguishing Electron-Poor and Electron-Rich Compounds
Several methods can be used to distinguish between electron-poor and electron-rich compounds:
Chemical Reactivity
Electron-poor compounds are more likely to react with electron-rich compounds, forming new bonds. This reactivity difference can be used to identify the electron richness or poorness of a compound.
Spectroscopic Techniques
Spectroscopic techniques, such as NMR and IR spectroscopy, can provide information about the electron distribution in a molecule. Electron-poor compounds tend to have higher chemical shifts in NMR spectra and stronger absorption bands in IR spectra.
Electrochemical Techniques
Electrochemical techniques, such as cyclic voltammetry, can measure the electron-donating or electron-accepting ability of a compound. Electron-poor compounds tend to have higher reduction potentials than electron-rich compounds.
Significance of Electron Richness/Poorness

Electron richness or poorness plays a crucial role in chemical reactions. Electron-poor compounds are often electrophiles, which are attracted to electron-rich nucleophiles. This interaction drives many organic reactions, such as nucleophilic addition and substitution reactions.Electron richness or poorness also affects the reactivity of compounds in redox reactions.
Electron-poor compounds are more likely to be oxidized, while electron-rich compounds are more likely to be reduced. Understanding electron richness or poorness is essential for predicting the reactivity and behavior of chemical compounds.
Expert Answers: Divide The Compounds Below Into Electron-poor And Electron-rich Groups
What is the significance of electron richness/poorness in chemical reactions?
Electron richness/poorness significantly affects the reactivity of compounds. Electron-rich compounds tend to be more reactive as nucleophiles, while electron-poor compounds are more reactive as electrophiles.
How can we distinguish between electron-poor and electron-rich compounds?
Several methods can be used to distinguish between electron-poor and electron-rich compounds, including electronegativity, oxidation state, and molecular orbital theory.